Shaowei Guo, Idan Redenski, Shira Landau, Ariel Szklanny, Uri Merdler, and Shulamit Levenberg*
Dr. S. Guo, Dr. I. Redenski, Dr. S. Landau, A. Szklanny, U. Merdler, Prof. S. Levenberg
Faculty of Biomedical Engineering, Technion-Israel Institute of Technology, Haifa 3200003, Israel.
Dr. S. Guo
The First Affiliated Hospital, Shantou University Medical College, Shantou 515000, China.
Dr. Shaowei Guo’s email: 07swguo1@stu.edu.cn; mobile: 13923961086
* Corresponding author: Shulamit Levenberg Email: shulamit@bm.technion.ac.il
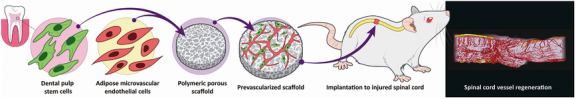
Background: The regeneration of injured spinal cord is hampered by the lack of vascular supply and neurotrophic support. Transplanting tissue engineered constructs with developed vascular networks and neurotrophic factors, and further understanding the pattern of vessel growth in the remodeled spinal cord tissue are greatly desired. Human dental pulp stem cells (DPSCs) are a source of stem cells that originate from migrating neural crest cells and reside in the dental pulp cavity. Their neurotrophic and angiogenic potentials bear promising clinical tissue regeneration implications for central nervous system disorders. In previous work, we engineered vascular networks on biocompatible and biodegradable poly(l-lactic acid)(PLLA)/polylactic-glycolic acid (PLGA) scaffolds, using a co-culture of endothelial cells and support cells. To this end, in this study, we aimed to fabricate highly vascularized scaffolds embedded with human dental pulp stem cells (DPSCs), which possess paracrine-mediated angiogenic and neuroregenerative potentials, to repair damaged spinal cord tissues.
Methods: We co-seeded an optimized ratio and density of DPSCs and human adipose microvascular endothelial cells on 3D PLLA/PLGA scaffolds, and compared the quantity and quality of vessel networks formed in this system with other pre-existing co-culture systems. In addition, the neurotrophic effect of those DPSC-embedded, prevascularized scaffolds were assessed on primary neurons. Next, in the rat complete spinal cord transection model, the DPSC-embedded, prevascularized scaffolds were implanted, followed by immunostaining, behavioral studies, neuroanatomic tracing, 3D microCT imaging and novel morphometric analysis of neo-vasculatures in the remodeled spinal cord tissues.
Results: The DPSC co-culture system outperformed other co-culture systems in promoting vessel formation on 3D scaffolds. Additionally, the highly vascularized scaffolds could also exert neurotrophic effect on primary neurons. The implantation of those scaffolds to the injured spinal cords promoted revascularization, as well as axon regeneration, myelin deposition, and sensory recovery. Furthermore, 3D microCT imaging and novel morphometric analysis on the remodeled spinal cord tissue demonstrated substantial regenerated vessels, more significantly in the sensory tract regions, which correlated with behavioral recovery following prevascularization treatment.
Conclusions: In conclusion, this work demonstrated the neuroregenerative and angiogenic potential of bioengineered prevascularized DPSC-embedded constructs, in a rat complete spinal cord transection model. Our results suggest the feasibility of harnessing prevascularization within stem cell-embedded constructs for augmenting and modulating SCI repair. In addition, we showed, for the first time, the ability to visualize and extrapolate vessel volumetric data in different tract regions after implantation of prevascularized scaffolds, which has deepened our understanding of the role of neovessels in functional outcomes. The approach used will likely advance the 3D analysis and interpretation of vessel formation in specific regions and the understanding of its therapeutic contribution in large-volume samples, such as primate spinal cord and brain.
