Bing Wen1,2, Li-Yan Xu1,3* and En-Min Li1,2*
1The Key Laboratory of Molecular Biology for High Cancer Incidence Coastal Chaoshan Area, Shantou University Medical College, Shantou 515041, Guangdong, P.R. China
2Department of Biochemistry and Molecular Biology, Shantou University Medical College, Shantou 515041, Guangdong, P.R. China
3Institute of Oncologic Pathology, Shantou University Medical College, Shantou 515041, Guangdong, P.R. China
*Corresponding authors: Dr. En-Min Li, Department of Biochemistry and Molecular Biology, Shantou University Medical College, 22 Xinling Road, Shantou 515041, China. Phone: 86-754-88900413, Fax: 86-754-88900847, E-mail: nmli@stu.edu.cn. Correspondence may also be addressed to Dr. Li-Yan Xu (E-mail: lyxu@stu.edu.cn)
第一作者:文兵;通讯地址:广东省汕头市金平区新陵路22号汕头大学医学院;单位名称:汕头大学医学院;身份信息:汕头大学医学院2019级生物化学与分子生物学在读博士,E-mail: 16bwen@stu.edu.cn,电话:13531151848。个人简历:文兵,男,江西省赣州市人,本科毕业于南昌大学生命科学学院生物技术专业,硕士毕业于汕头大学医学院生物化学与分子生物学专业,现博士就读于汕头大学医学院生物化学与分子生物学专业,研究方向为食管癌发生发展的分子机制。
通讯作者1:李恩民;通讯地址:广东省汕头市金平区新陵路22号汕头大学医学院;单位名称:汕头大学医学院;身份信息:汕头大学医学院教授,博导;E-mail: nmli@stu.edu.cn.电话:13809291605。个人简历:李恩民教授,医学生物化学与分子生物学专业,博士,二级教授,博导;汕头大学医学院基础医学研究所所长,汕头大学医学院生物医学研究中心主任,汕头大学医学院生物化学与分子生物学教研室主任,潮汕沿海地区高发肿瘤分子生物学广东省高校重点实验室主任,国家自然科学基金医学部会评专家,广东省国家级千百十工程人才,全国优秀教师,广东省教学名师,广东省五一劳动奖章获得者,南粤优秀教师,汕头市优秀教师,中国抗癌协会肿瘤转移专业委员会常委,中国生物化学与分子生物学会基础医学专业分会委员,广东省生物化学与分子生物学会副理事长,美国西南医学中心高级访问学者。作为通讯作者,在Cancer Res、Clin Cancer Res、J Pathol、Am J Pathol、Int J Cancer、Carcinogenesis、Mol Oncol和Biochem J等主流学术期刊杂志上发表研究论文;同时,作为共同第一作者,在Nature杂志上发表研究论文;所发表的190余篇SCI研究论文,累计他引4800余次。
通讯作者2:许丽艳;通讯地址:广东省汕头市金平区新陵路22号汕头大学医学院;单位名称:汕头大学医学院;身份信息:汕头大学医学院研究员,博导;E-mail: lyxu@stu.edu.cn.电话:13829490141。个人简历:许丽艳,汕头大学医学院,特聘教授/研究员,博士,博士研究生导师,汕头大学医学院肿瘤研究中心主任,汕头大学基础医学学科带头人,主要研究领域肿瘤分子病理学,中国抗癌协会整合肿瘤分会常务委员,中国抗癌协会肿瘤转移专业委员会委员,广东省抗癌协会肿瘤转移专业委员会副主任委员,广东省生物化学与分子生物学学会常务理事,潮汕沿海地区高发肿瘤分子生物学广东省高校重点实验室研究方向一负责人,广东省“千百十人才工程”省级培养对象,医学院校8年制/7年制国家规划教材《生物信息学》编委,汕头市优秀教师。作为通讯作者,在Nucleic Acids Res、Am J Gastroenterol、J Biol Chem、Cell Mol Life Sci、Cancer Prev Res、BBA-Mol Cell Res、Int J Cancer、J Proteomics和Br J Cancer等主流学术期刊杂志上发表研究论文;所发表的190余篇SCI研究论文,累计他引4800余次;同时获得中国发明专利8项。
注:本论文已发表于BBA-Reviews on Cancer(一年内),DOI: 10.1016/j.bbcan.2020.188435
Abstract
Lysyl oxidase-like 2 (LOXL2) is a copper and lysine tyrosyl-quinone (LTQ)-dependent amine oxidase belonging to the lysyl oxidase (LOX) family, the canonical function of which is to catalyze the crosslinking of elastin and collagen in the extracellular matrix (ECM). Many studies have revealed that the aberrant expression of LOXL2 in multiple cancers is associated with epithelial-mesenchymal transition (EMT), metastasis, poor prognosis, chemoradiotherapy resistance, and tumor progression. LOXL2 is regulated in many ways, such as transcriptional regulation, alternative splicing, microRNA regulation, posttranslational modification, and cleavage. Beyond affecting the extracellular environment, various intracellular roles, such as oxidation and deacetylation activities in the nucleus, have been reported for LOXL2. Additionally, LOXL2 contributes to tumor cell invasion by promoting cytoskeletal reorganization. Targeting LOXL2 has become a potential therapeutic strategy to combat many types of cancers. Here, we provide an overview of the regulation and downstream effectors of LOXL2 and discuss the intracellular role of LOXL2 in cancer.
Key words
LOXL2, oxidation, regulation, cytoskeleton, cancer
1. Introduction
The lysyl oxidase (LOX) family consists of five members: lysyl oxidase (LOX), lysyl oxidase-like 1 (LOXL1), lysyl oxidase-like 2 (LOXL2), lysyl oxidase-like 3 (LOXL3) and lysyl oxidase-like 4 (LOXL4), which are copper and lysine tyrosyl-quinone (LTQ)-dependent amine oxidases[1-5]. The canonical function of LOX is to catalyze the crosslinking of elastin and collagen, which is essential for the remodeling and stabilization of the extracellular matrix (ECM)[6, 7]. The human LOXL2 gene maps to chromosome 8p21.3 and encodes a 774 amino acid protein[8]. The LOXL2 protein contains a signal peptide and four scavenger receptor cysteine-rich (SRCR) domains in its N-terminus and possesses a conserved LOX catalytic domain in its C- terminus [9].
In 2003, Akiri et al. demonstrated for the first time that LOXL2 could promote tumor fibrosis and enhance the malignancy of breast cancer[10]. Since then, LOXL2 has attracted increasing attention because of its intricate and critical role in tumor progression. LOXL2 is abnormally overexpressed in numerous cancers (Table 1). In head and neck squamous carcinoma[11], breast cancer[12], lung cancer[13], colon cancer[14], gastric cancer[15], cervical cancer[16], liver cancer[17] and esophageal cancer[18], the high expression of LOXL2 is associated with reduced survival time and poor prognosis. LOXL2 promotes the proliferation, migration, invasion, and metastasis of tumors[15, 16, 19-33], leading to malignant progression of cancer. LOXL2 accelerates cancer progression by promoting tumor-related angiogenesis[21, 34, 35]. LOXL2 mediates the crosstalk of cancer cells and fibroblasts[14, 36]. LOXL2 changes the tumor microenvironment and then facilitates cancer metastasis[25, 37]. Additionally, LOXL2 is a key driver of epithelial-mesenchymal transition (EMT) in cancer[16, 27, 38-40]. Furthermore, high expression of LOXL2 reduces the sensitivity of cancer cells to chemoradiotherapy[41, 42]. LOXL2 is regulated at multiple levels, such as transcriptional regulation[43-46], alternative splicing[47-49], microRNA regulation[50-52], posttranslational modification[53], and extracellular protease cleavage[54, 55]. Hypoxia, extracellular ATP, fibroblasts, ECM stiffness, and other factors can also affect the expression of LOXL2[56-59]. Additionally, LOXL2 is closely related to cytoskeletal reorganization in a variety of cancer cells, and LOXL2 mediates the phosphorylation of ezrin (EZR) at T567 by protein kinase C alpha type (PKCα) in esophageal squamous cell carcinoma (ESCC)[30]. In short, LOXL2 is a multifunctional enzyme involved in extensive cellular processes in cancer.
This review discusses the regulation and downstream effectors of LOXL2 in cancers. In addition, we highlight recent advances in our understanding of the nonamine oxidase functions of LOXL2 and discuss the reorganization of the cytoskeleton mediated by LOXL2 based on results from current research.
2. Regulation of LOXL2
High expression of LOXL2 in tumors often indicates poor prognosis and more aggressive malignancy in cancer patients. In the last decade, many studies related to the regulation of LOXL2 have been published.
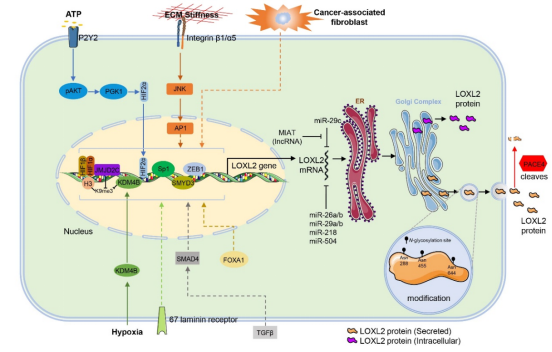
Figure 1. Regulation of LOXL2. LOXL2 gene is regulated by TGFβ/SMAD4 pathway, ATP/P2Y2/AKT/PGK1/HIF2α pathway, ECM/Integrinβ1/α5/C-Jun/AP1 pathway and Hypoxia/KDM4B pathway. CAFs, 67 laminin receptor and FOXA1 also induce LOXL2 expression. LOXL2 transcription can be regulated by several transcription factors and histone-modifying enzymes, including HIF1α, HIF2α, Sp1, ZEB1, JMJD2C, KDM4B, and SMYD3 (dotted lines indicate ambiguous links). Non-coding RNAs, including MIAT, miR-29a/b/c, miR-26a/b, miR-218, and miR-504 can also modulate LOXL2 mRNA levels. Secreted LOXL2 protein (orange) is modified by N-glycosylation at Asn288, Asn455 and Asn644 sites, while intracellular LOXL2 protein (purple) is not. LOXL2 is specifically cleaved by PACE4.
2.1. Transcriptional regulation of LOXL2
Several transcription factors contribute to LOXL2 transcription (Figure 1). Hypoxia of the tumor microenvironment is a salient feature in cancer progression and leads to the upregulation of hypoxia-related factors[60]. Hypoxia is one of the crucial regulatory factors of LOXL2 expression in cancer. In ovarian cancer, the histone demethylase lysine demethylase 4B (KDM4B) is upregulated by hypoxia and demethylates trimethylated histone H3 at lysine 9 (H3K9me3) at the LOXL2 gene promoter to increase the expression of LOXL2[45]. Hypoxia-inducible factor 1 (HIF1) and hypoxia-inducible factor 2 (HIF2) directly induce LOXL2 expression by binding to the LOXL2 gene promoter. In breast cancer, the histone demethylase Jumonji domain 2C (JMJD2C) is recruited by HIF1α to the LOXL2 promoter region. JMJD2C can inhibit the methylation of histone H3 at lysine 9 (H3K9), thereby upregulating the expression of LOXL2[43, 61]. Extracellular ATP can upregulate HIF2α through the P2Y2-mediated protein kinase B (AKT)-phosphoglycerate kinase 1 (PGK1) signaling pathway and increase the expression of LOXL2[59]. In addition, zinc finger E-box-binding homeobox 1 (ZEB1) binds to the promoter region of LOXL2 and directly regulates the expression of LOXL2[46]. In 2016, our laboratory found that the expression of the methyltransferase SMYD3 was abnormally increased in ESCC, and SMYD3 enhanced the transcription of LOXL2 by directly binding to the LOXL2 promoter[44]. Recently, Liu et al. predicted that the LOXL2 promoter contains specificity protein 1 (Sp1) and nuclear factor kappa B (NFκB) binding sites and demonstrated that Sp1 directly binds to the LOXL2 promoter region and enhances its proximal promoter activity[62]. Transforming growth factor beta 1 (TGF-β1) upregulates LOXL2 mRNA in a SMAD family member 4 (SMAD4)-dependent pattern[63]. In ESCC, forkhead box A1 (FOXA1) knockdown decreases the level of LOXL2 mRNA, but the mechanism is unclear[64], and in cholangiocarcinoma (CCA), 67 laminin receptor (67LR) increases the expression of LOXL2[57].
The crosstalk of tumor cells with cancer-associated fibroblasts (CAFs), ECM, and other tumor microenvironment components plays an essential role in accelerated cancer evolution[65]. Gene expression on tumor cells is influenced by the ECM and fibroblasts. CAFs can significantly upregulate the expression of LOXL2 in hepatocellular carcinoma (HCC) cells[56]. High matrix stiffness around tumor cells affects the biological response of tumor cells, regulates the expression of tumor-related genes, and promotes tumor invasion and metastasis. Matrix stiffness activates the integrin β1/α5/JNK/c-JUN signaling pathway and promotes the expression of the LOXL2 gene in HCC cells[58]. On the other hand, tumor cell secreted-LOXL2 mediates ECM remodeling and CAF activation[36, 66]. LOXL2 seems to be a “communicator” between tumor cells and the tumor microenvironment.
2.2. Posttranscriptional regulation of LOXL2
Alternative splicing offers the possibility of generating diversity at the RNA and protein levels from a limited number of loci in the genome[67]. To date, there have been few reports on the selective splicing of LOXL2. As early as 2014, our laboratory first identified and analyzed two splice variants of LOXL2 in ESCC, LOXL2-Δe13 and LOXL2-Δ72, which were different from wild-type LOXL2 (LOXL2-WT)[47, 48](Figure 2). Due to the deletion of exon 13 in LOXL2-Δe13 mRNA, 45 amino acid residues were truncated at the C-terminus of the LOXL2-Δe13 protein. Intriguingly, although the deamination activity of LOXL2-Δe13 is partially impaired, LOXL2-Δe13 possesses a stronger oncogenic function in ESCC cells than LOXL2-WT[48] or LOXL2-Δ72, a LOXL2 splice variant that lacks 72 nucleotides in exon 5. Protein-protein interaction (PPI) network analysis revealed that both LOXL2-Δe13 and LOXL2-Δ72 might have specific biological roles and molecular mechanisms that differ from those of LOXL2-WT[47, 68]. Recently, Liu et al. found a new LOXL2 splice variant (LOXL2-Var) in human papillomavirus (HPV)-negative head and neck squamous cell carcinoma (HNSCC). In LOXL2-Var mRNA, an additional 120 bp exon is inserted between canonical exons 1 and 2, which are noncoding, and a novel variant different from the 5’ untranslated region (UTR) of LOXL2-WT is formed(Figure 2). LOXL2-Var is highly expressed in HPV-negative HNSCC, promotes tumor progression after it is overexpressed in HPV-negative HNSCC cells, and activates the focal adhesion kinase (FAK)/AKT signaling pathway[49]. LOXL2-Δe13 and LOXL2-Δ72 may independently perform partial work of LOXL2 in cancer, and together with LOXL2-WT, they contribute to the malignant progression of the cancer.
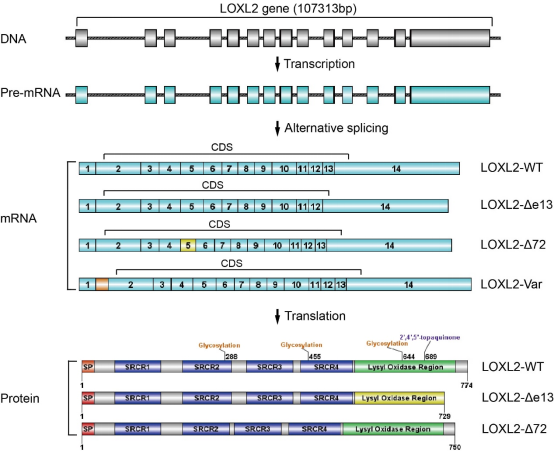
Figure 2. LOXL2 mRNA alternative splicing. The human LOXL2 gene contains 14 exons, and alternative splicing forms four different mRNA isoforms: LOXL2-WT, LOXL2-Δe13, LOXL2-Δ72, and LOXL2-Var. The translation of LOXL2-WT and LOXL2-Var mRNA produces the same LOXL2 protein (wild-type). LOXL2-Δe13 and LOXL2-Δ72 mRNA are translated into different protein compared with LOXL2-WT.
MicroRNA regulation of LOXL2 has also been elucidated in a variety of tumors. In HNSCC, miR-26a/b, miR-29a/b/c, and miR-218 significantly inhibit HNSCC metastasis by directly downregulating LOXL2 mRNA[50]. In lung squamous cell carcinoma (LSCC), miR-29a/b/c binds to the 3’ UTR of LOXL2 and prevents LOXL2 transcription, consequently inhibiting cancer cell migration and invasion[69]. Kamikawaji et al. also demonstrated a direct negative regulation between miR-29a and LOXL2 in lung cancer[70]. Importantly, the long noncoding RNA myocardial infarction associated transcript (MIAT) can up-regulate LOXL2 expression by competitively binding and inhibiting miR-29c in clear cell renal cell carcinoma (ccRCC)[51]. MiR-504 is a tumor suppressor in non-small cell lung cancer (NSCLC) and acts in part by downregulating LOXL2 by binding to the 3’ UTR of LOXL2[52].
2.3. Posttranslational regulation of LOXL2
An imbalance of protein glycosylation is closely related to human cancers[71]. N-glycosylation usually occurs only on membrane and secretory proteins, but not on cytoplasmic and nuclear proteins[72]. The recombinant human LOXL2 protein produced in Drosophila S2 cells is glycosylated (N-linked glycans) at N455 and N644, and the N-linked glycans at N455 and N644 are crucial to the stability and secretion of LOXL2[53]. Moreover, the recombinant human LOXL2 protein secreted by human embryonic kidney (HEK) cells is glycosylated (N-linked glycans) at N288, N455, and N644[73]. Overexpression of LOXL2 in breast cancer cell lines generates two forms of LOXL2: intracellular LOXL2 (~75-kDa) and secreted LOXL2 (~100-kDa). Intracellular LOXL2 is nonglycosylated and N-terminally processed, while secreted LOXL2 is glycosylated at N455 and N644[38]. After the treatment of alveolar rhabdomyosarcoma (ARMS) cells with the N-glycosylation inhibitor tunicamycin, the secretion of LOXL2 is inhibited, and the molecular weight of LOXL2 is decreased[31]. Trihydroxyphenolic compounds inhibit TGF-β1 responses and reduce fibrosis in lung cancer, and LOXL2 mediates this process. Trihydroxyphenolics irreversibly inhibit LOXL2 by inducing the auto-oxidation of LOXL2 K731, and trihydroxyphenolics are transformed into new metabolites that inhibit the TGF-β1 receptor (TβR) kinase[74]. Furthermore, extracellular LOXL2 undergoes hydrolysis catalyzed by paired basic amino acid cleaving enzyme 4 (PACE4), a significant protease responsible for cleaving LOXL2 at the K317↓- A318 site between the SRCR2 and SRCR3 domains. This process produces a hydrolyzed LOXL2 protein with a molecular weight of approximately 60 kDa and does not affect the catalytic activity of LOXL2[54]. In the ECM, the crosslinking of basement membrane collagen IV requires the proteolytic processing of LOXL2[55]. In endothelial cells, the hypoxia-induced long noncoding antisense (AS) transcript of GATA-binding factor 6 (GATA6; GATA6-AS) interacts with the LOXL2 protein and inhibits LOXL2-mediated deamination of trimethylated histone H3 at lysine 4 (H3K4me3), thereby regulating endothelial gene expression[75].
In summary, extracellular LOXL2 undergoes glycosylation and protease processing, which are essential for its secretion and ECM remodeling. In addition, the function of LOXL2 protein is regulated by its own oxidative modification and its molecular chaperones.
3. Downstream effectors of LOXL2 in cancer
The LOXL2 protein is widely distributed in the extracellular and intracellular compartments and in the nucleus, and it participates in multiple intracellular and extracellular processes through its diverse downstream effectors (Figure 3).
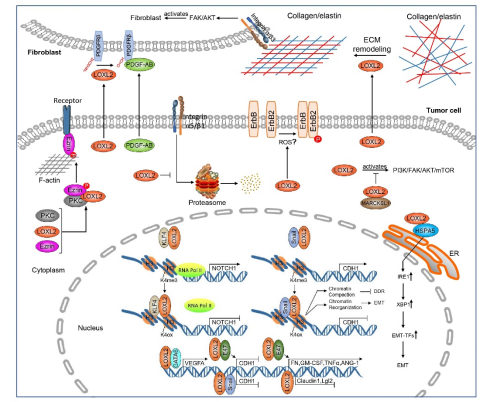
Figure 3. Downstream effectors of LOXL2 in cancer. Extracellular LOXL2 oxidizes the collagen and elastin of the extracellular matrix and the fibroblast membrane receptor PDGFRβ. There are several downstream effectors of LOXL2 in the cytoplasm, including Ezrin, integrin α5/β1, ErbB2, MARCKSL1, and HSPA5. In the nucleus, LOXL2 interacts with transcription factors such as KLF4, Snail1, GATA6, and E47. LOXL2 affects the transcription of downstream genes NOTCH1, CDH1, VEGFA, FN, GM-CSF, TNFα, ANG-1, claudin1, and Lgl2.
Extracellular LOXL2 regulates the tumor microenvironment. Oxidation is the usual function of LOXL2. LOXL2 catalyzes the oxidative deamination of lysine and hydroxylysine residues of collagen and tropoelastin in the ECM, which is followed by crosslinking between allysine and lysine residues of collagen and tropoelastin[66]. LOXL2 secreted by tumor cells promotes remodeling of the ECM, which in turn activates fibroblasts through integrin-mediated FAK/AKT signaling[36]. Additionally, extracellular LOXL2 secreted by oral tumor cells oxidizes lysine residues in platelet-derived growth factor receptor beta (PDGFRβ) on the oral fibroblast membrane. The oxidized PDGFRβ enhances the binding affinity of its ligand platelet-derived growth factor composed an A subunit and a B subunit (PDGF-AB). Subsequently, PDGF-AB secreted by tumor cells can more effectively stimulate PDGF signaling and increase the activation of extracellular signal-regulated kinase (ERK) and the proliferation of fibroblasts, eventually leading to the fibrosis and metastasis of oral cancer[37]. In breast cancer, LOXL2 promotes the expression and activity of tissue inhibitor of metalloproteinase-1 (TIMP1) and matrix metallopeptidase 9 (MMP9), thereby enhancing degradation and remodeling of the ECM, leading to breast cancer metastasis[20, 76].
LOXL2 also regulates the function of cytoplasmic proteins in cancer. LOXL2 promotes the expression of receptor activity modifying protein 3 (RAMP3) in several types of tumor cells, and the protumorigenic activity of LOXL2 is partly mediated by RAMP3[77]. Overexpression of LOXL2 in normal human mammary epithelial cells leads to oncogenic transformation and cancer progression. This abnormal change is caused by LOXL2-mediated activation of ErbB2 through reactive oxygen species (ROS) production[78]. In human breast carcinoma cell lines, LOXL2 interacts with myristoylated alanine-rich C-kinase substrate (MARCKS)-like protein 1 (MARCKSL1) through its N-terminal SRCR domain. LOXL2 promotes cancer cell growth by inhibiting apoptosis induced by MARCKSL1, which in turn, inhibits LOXL2-mediated FAK/AKT/mTOR signaling cascades[22]. LOXL2 decreases protease and proteasome-mediated protein degradation of integrin α5 (ITGA5) and integrin β1 (ITGB1) in ccRCC[28]. LOXL2 activates the inositol requiring enzyme 1α/β (IRE1)-X-box-binding protein 1 (XBP1) signaling pathway of the endoplasmic reticulum stress response (ERS) by directly interacting with heat shock protein family A (Hsp70) member 5 (HSPA5) and then induces the expression of the EMT transcription factors Snail family transcriptional repressor 1 (SNAI1), Snail family transcriptional repressor 2 (SNAI2), zinc finger E-box binding homeobox 2 (ZEB2) and transcription factor 3 (TCF3) to drive EMT[39]. In addition, LOXL2 forms a ternary complex with PKCα and EZR, enhancing the phosphorylation of EZR at T567 mediated by PKCα[30].
3.3. Nuclear and transcriptional targets
LOXL2 plays a vital role in transcriptional regulation within the nucleus in cancer. LOXL2 is located in the nucleus and is an EMT-promoting factor in many types of cancer. LOXL2 induces EMT by interacting with Snail1 and synergistically inhibiting the expression of E-cadherin[40]. However, the amine oxidase catalytic activity of LOXL2 is not required for EMT mediated by LOXL2 and Snail1. LOXL2 catalytically inactive mutants can also collaborate with Snail1 in cadherin-1 (CDH1) repression to promote EMT and activate the FAK/Src signaling pathway to boost EMT[79]. LOX- and LOXL2-mediated E-cadherin downregulation is activated by the upregulation of hypoxia-induced HIF1[80]. Expression of the intracellular nonglycosylated LOXL2 mutant (N455Q and N644Q) induces EMT by stabilizing the Snail1 transcription factor in MCF-7 cells, thereby downregulating E-cadherin, estrogen receptor-α and occludin, as well as upregulating vimentin, membrane type 1-matrix metalloproteinase (MT1-MMP), and fibronectin[38]. Snail1 is upregulated and binds to heterochromatin during EMT, and then LOXL2 is recruited to oxidize histone H3, resulting in heterochromatin reorganization, thereby promoting EMT[81]. LOXL2 demethylates H3K4me3 and converts H3K4me3 to oxidation of histone H3 at lysine 4 (H3K4ox) by an amino-oxidase reaction, which represses CDH1 gene transcription[82]. In triple-negative breast cancer, LOXL2-mediated H3K4 oxidation lowers chromatin accessibility, inhibits the DNA damage response (DDR), and reduces cancer cell sensitivity to anticancer drugs[41]. LOXL2 interacts with Krüppel-like factor 4 (KLF4) and binds to the promoter of the neurogenic locus notch homolog protein 1(NOTCH1) gene to oxidize H3K4me3 and reduce the binding of RNA polymerase Ⅱ, thereby inhibiting the transcription of NOTCH1 and promoting the progression of squamous cell carcinoma (SCC)[83]. The basic helix-loop-helix (bHLH) transcription factor E47 is an EMT inducer. LOXL2 interacts with E47 and synergistically inhibits CDH1 transcription by directly binding to the promoter region. Primary tumors accelerate distal metastasis by guiding bone marrow-derived cells (BMDCs) to colonize target organs before the arrival of cancer cells[84]. Primary tumors induce ECM remodeling by secreting cytokines to constitute the tumor microenvironment[85]. Hypoxia-induced LOXL2 contributes to the recruitment of BMDCs by breast carcinoma cells.[86] LOXL2 promotes premetastatic niche by recruiting BMDCs and enhancing cytokine expression. LOXL2 and E47 can regulate the transcription of fibronectin and cytokines tumor necrosis factor alpha (TNFα), angiopoietin-1 (ANG-1), and granulocyte-macrophage colony-stimulating factor (GM-CSF) in primary xenografted tumors[23]. Moreover, LOXL2 maintains the mesenchymal phenotype of basal-like carcinoma cells through downregulation of Lgl2 and claudin-1 transcription and disorganization of cell polarity and tight junction complexes[33]. In CCA, LOXL2 interacts with GATA6 and directly binds to the vascular endothelial growth factor A (VEGFA) promoter to up-regulate VEGFA transcription, inducing angiogenesis[35].
4. Nonamine oxidase functions of LOXL2
The familiar and canonical role of LOXL2 depends on its amine-oxidase activity. However, LOXL2 plays an essential role in diverse biological processes that are independent of its amine oxidase activity, indicating that LOXL2 contains novel functions beyond its amine oxidase function. Unexpectedly, catalytically-inactive mutants of LOXL2 can induce EMT by collaborating with Snail1 and activating the FAK/Src signaling pathway[79]. LOXL2 activates the IRE1-XBP1 signaling pathway of the ERS by interacting directly with HSPA5 and then inducing EMT. Interestingly, an inactive LOXL2 mutant lacking 120 amino acids in the catalytic domain can also exceed the effect of LOXL2-WT[39]. LOXL2-Δe13, a nonenzymatic, spliced isoform of LOXL2, has been shown to promote the migration, invasion, and metastasis of ESCC cells more strongly than LOXL2-WT[30, 48]. In ARMS, LOXL2 promotes malignant tumor progression independent of its enzyme activity[31]. In any case, the amine oxidase catalytic activity-independent functions of LOXL2 have been elucidated in many models, but the mechanism has not yet been determined.
The SRCR domains of LOXL2-4 possess an unexpected function that differs from the C-terminal catalytic domain. In 2016, Ma et al. discovered that the N-terminal SRCR domain of LOXL3 has deacetylase activity and deacetylated signal transducer and activator of transcription 3 (STAT3), and LOXL2 could deacetylate Snail1 in a manner that is dependent on its N-terminal SRCR repeats[87]. However, this effect is limited to the HEK293 model, but its deacetylase activity in cancer cells has not been observed. In 2018, the Yigong Shi laboratory analyzed the crystal structure of LOXL2 (318-774 aa, containing the SRCR3, SRCR4, and LOX catalytic domains), which is triangular in shape, with the SRCR3, SRCR4 and LOX catalytic domains each occupying one angle (PDB ID: 5ZE3)[88]. In addition, our group found that LOXL2 can reduce the glycolytic-associated protein acetylation levels in ESCC (Jiao et al., unpublished data). The role of LOXL2 as a bifunctional enzyme in tumors deserves further exploration. Although the deacetylating activity of LOXL2 has not been observed in cancer, the versatility of LOXL2 may explain why its cancer-promoting effects are partially independent of amino oxidase catalytic activity. On the other hand, the SRCR domain is a widespread and highly conserved protein module, consisting of 90-115 amino acids, which usually mediates PPIs[73]. LOXL2 interacts with its partners through the N-terminal SRCR domain in breast cancer and CCA[21, 34]. Therefore, LOXL2 may also act as a “scaffold” to help cancer-associated proteins come closer in proximity to each other, thereby driving cancer progression.
5. LOXL2 and cytoskeleton reorganization
In recent years, studies between LOXL2 and the cytoskeleton have gradually become a new research focus of LOXL2. LOXL2 induced by hypoxia enhances cytoskeletal remodeling and the accumulation of focal adhesion-related molecules in HCC cells, promoting the invasion and metastasis of HCC cells[29]. LOXL2 knockdown reduced the formation of stress fibers and focal adhesion in ccRCC cells[28]. In 2017, our laboratory discovered that LOXL2 and F-actin were colocalized in the cytoplasm near the cell membrane of ESCC cell lines by immunofluorescence. In ESCC patients, the LOXL2 expression level is positively correlated with actin cytoskeleton gene regulation [89]. The number and length of filopodia in ESCC cells are reduced by LOXL2 knockdown[30]. Moreover, four actin-binding proteins identified by mass spectrometry, EZR, fascin (FSCN1), heat shock protein beta-1 (HSPB1), and tropomodulin 3 (TMOD3), interact with LOXL2 in ESCC cell lines[30]. LOXL2 enhances PKCα-mediated phosphorylation of EZR at T567 and promotes cytoskeletal remodeling and tumor metastasis in ESCC[30]. Recently, it has been reported that LOXL2 interacts with the intermediate filament protein vimentin, which increases the cleavage of vimentin and promotes ARMS cell motility[31].
6. Conclusions and perspectives
LOXL2 promotes EMT and participates in the tumor microenvironment composition, which is an “accomplice” of tumor progression and metastasis, and its high expression is a risk factor for patients with many cancers. LOXL2 expression in cancer is regulated by hypoxia and other factors. In addition to playing an extracellular role in the tumor microenvironment, LOXL2 controls transcriptional regulation in the nucleus.
The LOX catalytic inhibitor β-aminopropionitrile (BAPN) significantly inhibits cancer cell invasion in breast cancer and uveal melanoma[90, 91], suggesting that LOX could be a potential antitumor target. In 2010, the group of V. Smith developed a monoclonal antibody inhibitor of LOXL2, AB0023, which specifically binds to the fourth SRCR domain of LOXL2[92]. In preclinical fibrosis and metastatic xenograft models, AB0023 can improve pulmonary fibrosis and inhibit tumor metastasis[93]. These studies provide the basis for further LOXL2-targeted therapy. Simtuzumab, a humanized IgG4 monoclonal antibody to LOXL2, has entered clinical trials for fibrosis[94, 95], colorectal adenocarcinoma[96], and pancreatic adenocarcinoma[97] but has shown unsatisfactory performance. Monoclonal antibody inhibitors can target only extracellular LOXL2, which limits their ability to inhibit intracellular LOXL2. Therefore, small molecule inhibitors may be a better choice for LOXL2-targeted cancer drug discovery. In 2017, Hutchinson et al. identified an efficient small molecular inhibitor of LOXL2: (2-chloropyridin-4-yl)methanamine 20. Compared with LOX and three other amine oxidases (monoamine oxidase A (MAO-A), monoamine oxidase B (MAO-B) and semicarbazide-sensitive amine oxidase (SSAO)), (2-chloropyridin-4-yl) methanamine 20 can specifically inhibit LOXL2[98]. Racemic-trans-(3-((4-(aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)oxy)phenyl)(3-fluoro-4-hydroxypyrrolidin-1-yl)methanone 28, a potent irreversible inhibitor of LOXL2 that is highly selective over LOX and other amine oxidases, can significantly inhibit fibrosis in a mouse lung bleomycin model[99]. In 2019, Schilter et al. developed the LOXL2/3 small molecule inhibitor PXS‐5153A, which can significantly improve fibrosis[100]. Additionally, LOXL2 inhibition caused by PXS compounds decreases the proliferation, migration, invasion, tumor angiogenesis and tumor metastasis in breast cancer cell models[101]. The development and application of LOXL2 small molecule inhibitors in cancer therapy are worth exploring.
In conclusion, we summarized the regulation mode and downstream targets of LOXL2 and discussed the nonamine oxidase action of LOXL2 and the newly discovered role of LOXL2 in cytoskeletal rearrangement. We provide a perspective for LOXL2-targeted drug development and clinical trials.
Acknowledgements
We are very thankful to Professor Stanley Li Lin of Shantou University Medical College for editing the manuscript. This work was supported by grants from the National Natural Science Foundation of China (81472613).
Abbreviations
ARMS, alveolar rhabdomyosarcoma; CAFs, cancer-associated fibroblasts; ccRCC, clear cell renal cell carcinoma; CDH1, cadherin-1 (the gene encoding E-cadherin); ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; ESCC, esophageal squamous cell carcinoma; FAK, focal adhesion kinase; GATA6, GATA-binding factor 6; H3K4, histone H3 lysine 4; H3K9, histone H3 lysine 9; HCC, hepatocellular carcinoma; HIF, hypoxia-inducible factor; HNSCC, head and neck squamous cell carcinoma; LOX, lysyl oxidase; LOXL2, lysyl oxidase-like 2; LOXL3, lysyl oxidase-like 3; LSCC, lung squamous cell carcinoma; LTQ, lysine tyrosyl-quinone; NSCLC, non-small cell lung cancer; PKCα, protein kinase C alpha type; SCC, squamous cell carcinoma; SRCR, scavenger receptor cysteine-rich; TGF-β1, transforming growth factor-beta-1; UTR, untranslated region; ZEB1, zinc finger E-box-binding homeobox 1
Reference
[1] K. Kenyon, W.S. Modi, S. Contente, R.M. Friedman, A novel human cDNA with a predicted protein similar to lysyl oxidase maps to chromosome 15q24-q25, The Journal of biological chemistry, 268 (1993) 18435-18437.
[2] Y. Kim, C.D. Boyd, K. Csiszar, A new gene with sequence and structural similarity to the gene encoding human lysyl oxidase, The Journal of biological chemistry, 270 (1995) 7176-7182.
[3] H. Saito, J. Papaconstantinou, H. Sato, S. Goldstein, Regulation of a novel gene encoding a lysyl oxidase-related protein in cellular adhesion and senescence, The Journal of biological chemistry, 272 (1997) 8157-8160.
[4] Y. Huang, J. Dai, R. Tang, W. Zhao, Z. Zhou, W. Wang, K. Ying, Y. Xie, Y. Mao, Cloning and characterization of a human lysyl oxidase-like 3 gene (hLOXL3), Matrix biology : journal of the International Society for Matrix Biology, 20 (2001) 153-157.
[5] J.M. Maki, K.I. Kivirikko, Cloning and characterization of a fourth human lysyl oxidase isoenzyme, The Biochemical journal, 355 (2001) 381-387.
[6] S.X. Wang, M. Mure, K.F. Medzihradszky, A.L. Burlingame, D.E. Brown, D.M. Dooley, A.J. Smith, H.M. Kagan, J.P. Klinman, A crosslinked cofactor in lysyl oxidase: redox function for amino acid side chains, Science, 273 (1996) 1078-1084.
[7] L.I. Smith-Mungo, H.M. Kagan, Lysyl oxidase: properties, regulation and multiple functions in biology, Matrix biology : journal of the International Society for Matrix Biology, 16 (1998) 387-398.
[8] C. Jourdan-Le Saux, O. Le Saux, T. Donlon, C.D. Boyd, K. Csiszar, The human lysyl oxidase-related gene (LOXL2) maps between markers D8S280 and D8S278 on chromosome 8p21.2-p21.3, Genomics, 51 (1998) 305-307.
[9] K. Csiszar, Lysyl oxidases: a novel multifunctional amine oxidase family, Progress in nucleic acid research and molecular biology, 70 (2001) 1-32.
[10] G. Akiri, E. Sabo, H. Dafni, Z. Vadasz, Y. Kartvelishvily, N. Gan, O. Kessler, T. Cohen, M. Resnick, M. Neeman, G. Neufeld, Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo, Cancer research, 63 (2003) 1657-1666.
[11] H. Peinado, G. Moreno-Bueno, D. Hardisson, E. Perez-Gomez, V. Santos, M. Mendiola, J.I. de Diego, M. Nistal, M. Quintanilla, F. Portillo, A. Cano, Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas, Cancer research, 68 (2008) 4541-4550.
[12] S.G. Ahn, S.M. Dong, A. Oshima, W.H. Kim, H.M. Lee, S.A. Lee, S.H. Kwon, J.H. Lee, J.M. Lee, J. Jeong, H.D. Lee, J.E. Green, LOXL2 expression is associated with invasiveness and negatively influences survival in breast cancer patients, Breast cancer research and treatment, 141 (2013) 89-99.
[13] P. Zhan, X.J. Lv, Y.N. Ji, H. Xie, L.K. Yu, Increased lysyl oxidase-like 2 associates with a poor prognosis in non-small cell lung cancer, The clinical respiratory journal, 12 (2018) 712-720.
[14] S. Torres, I. Garcia-Palmero, M. Herrera, R.A. Bartolome, C. Pena, M.J. Fernandez-Acenero, G. Padilla, A. Pelaez-Garcia, M. Lopez-Lucendo, R. Rodriguez-Merlo, A. Garcia de Herreros, F. Bonilla, J.I. Casal, LOXL2 Is Highly Expressed in Cancer-Associated Fibroblasts and Associates to Poor Colon Cancer Survival, Clinical cancer research : an official journal of the American Association for Cancer Research, 21 (2015) 4892-4902.
[15] L. Peng, Y.L. Ran, H. Hu, L. Yu, Q. Liu, Z. Zhou, Y.M. Sun, L.C. Sun, J. Pan, L.X. Sun, P. Zhao, Z.H. Yang, Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway, Carcinogenesis, 30 (2009) 1660-1669.
[16] J. Tian, H.X. Sun, Y.C. Li, L. Jiang, S.L. Zhang, Q. Hao, LOXL 2 Promotes The Epithelial-Mesenchymal Transition And Malignant Progression Of Cervical Cancer, OncoTargets and therapy, 12 (2019) 8947-8954.
[17] J. Choi, T. Chung, H. Rhee, Y.J. Kim, Y. Jeon, J.E. Yoo, S. Noh, D.H. Han, Y.N. Park, Increased Expression of the Matrix-Modifying Enzyme Lysyl Oxidase-Like 2 in Aggressive Hepatocellular Carcinoma with Poor Prognosis, Gut and liver, 13 (2019) 83-92.
[18] T.Y. Li, L.Y. Xu, Z.Y. Wu, L.D. Liao, J.H. Shen, X.E. Xu, Z.P. Du, Q. Zhao, E.M. Li, Reduced nuclear and ectopic cytoplasmic expression of lysyl oxidase-like 2 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma, Human pathology, 43 (2012) 1068-1076.
[19] F. Salvador, A. Martin, C. Lopez-Menendez, G. Moreno-Bueno, V. Santos, A. Vazquez-Naharro, P.G. Santamaria, S. Morales, P.R. Dubus, L. Muinelo-Romay, R. Lopez-Lopez, J.C. Tung, V.M. Weaver, F. Portillo, A. Cano, Lysyl Oxidase-like Protein LOXL2 Promotes Lung Metastasis of Breast Cancer, Cancer research, 77 (2017) 5846-5859.
[20] H.E. Barker, J. Chang, T.R. Cox, G. Lang, D. Bird, M. Nicolau, H.R. Evans, A. Gartland, J.T. Erler, LOXL2-mediated matrix remodeling in metastasis and mammary gland involution, Cancer research, 71 (2011) 1561-1572.
[21] C. Wang, S. Xu, Y. Tian, A. Ju, Q. Hou, J. Liu, Y. Fu, Y. Luo, Lysyl Oxidase-Like Protein 2 Promotes Tumor Lymphangiogenesis and Lymph Node Metastasis in Breast Cancer, Neoplasia, 21 (2019) 413-427.
[22] B.R. Kim, S.M. Dong, S.H. Seo, J.H. Lee, J.M. Lee, S.H. Lee, S.B. Rho, Lysyl oxidase-like 2 (LOXL2) controls tumor-associated cell proliferation through the interaction with MARCKSL1, Cellular signalling, 26 (2014) 1765-1773.
[23] G. Canesin, E.P. Cuevas, V. Santos, C. Lopez-Menendez, G. Moreno-Bueno, Y. Huang, K. Csiszar, F. Portillo, H. Peinado, D. Lyden, A. Cano, Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: novel partners in E-cadherin repression and early metastasis colonization, Oncogene, 34 (2015) 951-964.
[24] Y. Kim, S. Roh, J.Y. Park, Y. Kim, D.H. Cho, J.C. Kim, Differential expression of the LOX family genes in human colorectal adenocarcinomas, Oncology reports, 22 (2009) 799-804.
[25] H. Kasashima, M. Yashiro, H. Kinoshita, T. Fukuoka, T. Morisaki, G. Masuda, K. Sakurai, N. Kubo, M. Ohira, K. Hirakawa, Lysyl oxidase-like 2 (LOXL2) from stromal fibroblasts stimulates the progression of gastric cancer, Cancer letters, 354 (2014) 438-446.
[26] R. Nishikawa, T. Chiyomaru, H. Enokida, S. Inoguchi, T. Ishihara, R. Matsushita, Y. Goto, I. Fukumoto, M. Nakagawa, N. Seki, Tumour-suppressive microRNA-29s directly regulate LOXL2 expression and inhibit cancer cell migration and invasion in renal cell carcinoma, FEBS letters, 589 (2015) 2136-2145.
[27] X. Hong, J.J. Yu, Silencing of lysyl oxidaselike 2 inhibits the migration, invasion and epithelialtomesenchymal transition of renal cell carcinoma cells through the Src/FAK signaling pathway, International journal of oncology, 54 (2019) 1676-1690.
[28] H. Hase, K. Jingushi, Y. Ueda, K. Kitae, H. Egawa, I. Ohshio, R. Kawakami, Y. Kashiwagi, Y. Tsukada, T. Kobayashi, W. Nakata, K. Fujita, M. Uemura, N. Nonomura, K. Tsujikawa, LOXL2 status correlates with tumor stage and regulates integrin levels to promote tumor progression in ccRCC, Molecular cancer research : MCR, 12 (2014) 1807-1817.
[29] C.C. Wong, A.P. Tse, Y.P. Huang, Y.T. Zhu, D.K. Chiu, R.K. Lai, S.L. Au, A.K. Kai, J.M. Lee, L.L. Wei, F.H. Tsang, R.C. Lo, J. Shi, Y.P. Zheng, C.M. Wong, I.O. Ng, Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma, Hepatology, 60 (2014) 1645-1658.
[30] X.H. Zhan, J.W. Jiao, H.F. Zhang, X.E. Xu, J.Z. He, R.L. Li, H.Y. Zou, Z.Y. Wu, S.H. Wang, J.Y. Wu, L.D. Liao, J.J. Wang, Y.W. Cheng, K. Zhang, G. Neufeld, L.Y. Xu, E.M. Li, LOXL2 Upregulates Phosphorylation of Ezrin to Promote Cytoskeletal Reorganization and Tumor Cell Invasion, Cancer research, 79 (2019) 4951-4964.
[31] O. Almacellas-Rabaiget, P. Monaco, J. Huertas-Martinez, S. Garcia-Monclus, M. Chicon-Bosch, S. Maqueda-Marcos, I. Fabra-Heredia, D. Herrero-Martin, S. Rello-Varona, E. de Alava, R. Lopez-Alemany, P.H. Giangrande, O.M. Tirado, LOXL2 promotes oncogenic progression in alveolar rhabdomyosarcoma independently of its catalytic activity, Cancer letters, 474 (2020) 1-14.
[32] K. Weidenfeld, S. Schif-Zuck, H. Abu-Tayeh, K. Kang, O. Kessler, M. Weissmann, G. Neufeld, D. Barkan, Dormant tumor cells expressing LOXL2 acquire a stem-like phenotype mediating their transition to proliferative growth, Oncotarget, 7 (2016) 71362-71377.
[33] G. Moreno-Bueno, F. Salvador, A. Martin, A. Floristan, E.P. Cuevas, V. Santos, A. Montes, S. Morales, M.A. Castilla, A. Rojo-Sebastian, A. Martinez, D. Hardisson, K. Csiszar, F. Portillo, H. Peinado, J. Palacios, A. Cano, Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas, EMBO molecular medicine, 3 (2011) 528-544.
[34] B. Shao, X. Zhao, T. Liu, Y. Zhang, R. Sun, X. Dong, F. Liu, N. Zhao, D. Zhang, L. Wu, Y. Wang, M. Wang, J. Meng, X. Lin, B. Sun, LOXL2 promotes vasculogenic mimicry and tumour aggressiveness in hepatocellular carcinoma, Journal of cellular and molecular medicine, 23 (2019) 1363-1374.
[35] T. Peng, X. Deng, F. Tian, Z. Li, P. Jiang, X. Zhao, G. Chen, Y. Chen, P. Zheng, D. Li, S. Wang, The interaction of LOXL2 with GATA6 induces VEGFA expression and angiogenesis in cholangiocarcinoma, International journal of oncology, 55 (2019) 657-670.
[36] H.E. Barker, D. Bird, G. Lang, J.T. Erler, Tumor-secreted LOXL2 activates fibroblasts through FAK signaling, Molecular cancer research : MCR, 11 (2013) 1425-1436.
[37] F. Mahjour, V. Dambal, N. Shrestha, V. Singh, V. Noonan, A. Kantarci, P.C. Trackman, Mechanism for oral tumor cell lysyl oxidase like-2 in cancer development: synergy with PDGF-AB, Oncogenesis, 8 (2019) 34.
[38] H.J. Moon, J. Finney, L. Xu, D. Moore, D.R. Welch, M. Mure, MCF-7 cells expressing nuclear associated lysyl oxidase-like 2 (LOXL2) exhibit an epithelial-to-mesenchymal transition (EMT) phenotype and are highly invasive in vitro, The Journal of biological chemistry, 288 (2013) 30000-30008.
[39] E.P. Cuevas, P. Eraso, M.J. Mazon, V. Santos, G. Moreno-Bueno, A. Cano, F. Portillo, LOXL2 drives epithelial-mesenchymal transition via activation of IRE1-XBP1 signalling pathway, Scientific reports, 7 (2017) 44988.
[40] H. Peinado, M. Del Carmen Iglesias-de la Cruz, D. Olmeda, K. Csiszar, K.S. Fong, S. Vega, M.A. Nieto, A. Cano, F. Portillo, A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression, The EMBO journal, 24 (2005) 3446-3458.
[41] J.P. Cebria-Costa, L. Pascual-Reguant, A. Gonzalez-Perez, G. Serra-Bardenys, J. Querol, M. Cosin, G. Verde, R.A. Cigliano, W. Sanseverino, S. Segura-Bayona, A. Iturbide, D. Andreu, P. Nuciforo, C. Bernado-Morales, V. Rodilla, J. Arribas, J. Yelamos, A.G. de Herreros, T.H. Stracker, S. Peiro, LOXL2-mediated H3K4 oxidation reduces chromatin accessibility in triple-negative breast cancer cells, Oncogene, 39 (2020) 79-121.
[42] P. Xie, H. Yu, F. Wang, F. Yan, X. He, Inhibition of LOXL2 Enhances the Radiosensitivity of Castration-Resistant Prostate Cancer Cells Associated with the Reversal of the EMT Process, BioMed research international, 2019 (2019) 4012590.
[43] W. Luo, R. Chang, J. Zhong, A. Pandey, G.L. Semenza, Histone demethylase JMJD2C is a coactivator for hypoxia-inducible factor 1 that is required for breast cancer progression, Proceedings of the National Academy of Sciences of the United States of America, 109 (2012) E3367-3376.
[44] Y. Zhu, M.X. Zhu, X.D. Zhang, X.E. Xu, Z.Y. Wu, L.D. Liao, L.Y. Li, Y.M. Xie, J.Y. Wu, H.Y. Zou, J.J. Xie, E.M. Li, L.Y. Xu, SMYD3 stimulates EZR and LOXL2 transcription to enhance proliferation, migration, and invasion in esophageal squamous cell carcinoma, Human pathology, 52 (2016) 153-163.
[45] C. Wilson, L. Qiu, Y. Hong, T. Karnik, G. Tadros, B. Mau, T. Ma, Y. Mu, J. New, R.J. Louie, S. Gunewardena, A.K. Godwin, O.W. Tawfik, J. Chien, K.F. Roby, A.J. Krieg, The histone demethylase KDM4B regulates peritoneal seeding of ovarian cancer, Oncogene, 36 (2017) 2565-2576.
[46] D.H. Peng, C. Ungewiss, P. Tong, L.A. Byers, J. Wang, J.R. Canales, P.A. Villalobos, N. Uraoka, B. Mino, C. Behrens, Wistuba, II, R.I. Han, C.A. Wanna, M. Fahrenholtz, K.J. Grande-Allen, C.J. Creighton, D.L. Gibbons, ZEB1 induces LOXL2-mediated collagen stabilization and deposition in the extracellular matrix to drive lung cancer invasion and metastasis, Oncogene, 36 (2017) 1925-1938.
[47] B.L. Wu, H.Y. Zou, G.Q. Lv, Z.P. Du, J.Y. Wu, P.X. Zhang, L.Y. Xu, E.M. Li, Protein-protein interaction network analyses for elucidating the roles of LOXL2-delta72 in esophageal squamous cell carcinoma, Asian Pacific journal of cancer prevention : APJCP, 15 (2014) 2345-2351.
[48] G.Q. Lv, H.Y. Zou, L.D. Liao, H.H. Cao, F.M. Zeng, B.L. Wu, J.J. Xie, W.K. Fang, L.Y. Xu, E.M. Li, Identification of a novel lysyl oxidase-like 2 alternative splicing isoform, LOXL2 Deltae13, in esophageal squamous cell carcinoma, Biochemistry and cell biology = Biochimie et biologie cellulaire, 92 (2014) 379-389.
[49] C. Liu, T. Guo, A. Sakai, S. Ren, T. Fukusumi, M. Ando, S. Sadat, Y. Saito, J.A. Califano, A novel splice variant of LOXL2 promotes progression of human papillomavirus-negative head and neck squamous cell carcinoma, Cancer, 126 (2020) 737-748.
[50] I. Fukumoto, N. Kikkawa, R. Matsushita, M. Kato, A. Kurozumi, R. Nishikawa, Y. Goto, K. Koshizuka, T. Hanazawa, H. Enokida, M. Nakagawa, Y. Okamoto, N. Seki, Tumor-suppressive microRNAs (miR-26a/b, miR-29a/b/c and miR-218) concertedly suppressed metastasis-promoting LOXL2 in head and neck squamous cell carcinoma, Journal of human genetics, 61 (2016) 109-118.
[51] Y. Qu, H. Xiao, W. Xiao, Z. Xiong, W. Hu, Y. Gao, Z. Ru, C. Wang, L. Bao, K. Wang, H. Ruan, Z. Song, K. Chen, X. Zhang, H. Yang, Upregulation of MIAT Regulates LOXL2 Expression by Competitively Binding MiR-29c in Clear Cell Renal Cell Carcinoma, Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 48 (2018) 1075-1087.
[52] M.F. Ye, J.G. Zhang, T.X. Guo, X.J. Pan, MiR-504 inhibits cell proliferation and invasion by targeting LOXL2 in non small cell lung cancer, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 97 (2018) 1289-1295.
[53] L. Xu, E.P. Go, J. Finney, H. Moon, M. Lantz, K. Rebecchi, H. Desaire, M. Mure, Post-translational modifications of recombinant human lysyl oxidase-like 2 (rhLOXL2) secreted from Drosophila S2 cells, The Journal of biological chemistry, 288 (2013) 5357-5363.
[54] K. Okada, H.J. Moon, J. Finney, A. Meier, M. Mure, Extracellular Processing of Lysyl Oxidase-like 2 and Its Effect on Amine Oxidase Activity, Biochemistry, 57 (2018) 6973-6983.
[55] A.J. Lopez-Jimenez, T. Basak, R.M. Vanacore, Proteolytic processing of lysyl oxidase-like-2 in the extracellular matrix is required for crosslinking of basement membrane collagen IV, The Journal of biological chemistry, 292 (2017) 16970-16982.
[56] Z.Y. Lin, Y.H. Chuang, W.L. Chuang, Cancer-associated fibroblasts up-regulate CCL2, CCL26, IL6 and LOXL2 genes related to promotion of cancer progression in hepatocellular carcinoma cells, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 66 (2012) 525-529.
[57] J. Xu, D. Li, X. Li, Z. Liu, T. Li, P. Jiang, Q. He, F. Tian, Y. Gao, D. Wang, S. Wang, 67 laminin receptor promotes the malignant potential of tumour cells up-regulating lysyl oxidase-like 2 expression in cholangiocarcinoma, Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver, 46 (2014) 750-757.
[58] S. Wu, Q. Zheng, X. Xing, Y. Dong, Y. Wang, Y. You, R. Chen, C. Hu, J. Chen, D. Gao, Y. Zhao, Z. Wang, T. Xue, Z. Ren, J. Cui, Matrix stiffness-upregulated LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression and BMDCs recruitment to assist pre-metastatic niche formation, Journal of experimental & clinical cancer research : CR, 37 (2018) 99.
[59] H. Yang, Y.H. Geng, P. Wang, Y.T. Zhou, H. Yang, Y.F. Huo, H.Q. Zhang, Y. Li, H.Y. He, X.X. Tian, W.G. Fang, Extracellular ATP promotes breast cancer invasion and epithelial-mesenchymal transition via hypoxia-inducible factor 2alpha signaling, Cancer science, 110 (2019) 2456-2470.
[60] D.M. Gilkes, G.L. Semenza, D. Wirtz, Hypoxia and the extracellular matrix: drivers of tumour metastasis, Nature reviews. Cancer, 14 (2014) 430-439.
[61] M. Wang, X. Zhao, D. Zhu, T. Liu, X. Liang, F. Liu, Y. Zhang, X. Dong, B. Sun, HIF-1alpha promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment, Journal of experimental & clinical cancer research : CR, 36 (2017) 60.
[62] X. Liu, T. Liu, L. Hu, T. Jiang, H. Liu, Y. Wang, Y. Lei, J. Zhu, Y. Bu, Identification and characterization of the promoter of cancer-related gene LOXL2, Experimental cell research, 387 (2020) 111786.
[63] Z. Ezzoukhry, E. Henriet, L. Piquet, K. Boye, P. Bioulac-Sage, C. Balabaud, G. Couchy, J. Zucman-Rossi, V. Moreau, F. Saltel, TGF-beta1 promotes linear invadosome formation in hepatocellular carcinoma cells, through DDR1 up-regulation and collagen I cross-linking, European journal of cell biology, 95 (2016) 503-512.
[64] M. Sano, K. Aoyagi, H. Takahashi, T. Kawamura, T. Mabuchi, H. Igaki, Y. Tachimori, H. Kato, A. Ochiai, H. Honda, Y. Nimura, M. Nagino, T. Yoshida, H. Sasaki, Forkhead box A1 transcriptional pathway in KRT7-expressing esophageal squamous cell carcinomas with extensive lymph node metastasis, International journal of oncology, 36 (2010) 321-330.
[65] M. Nazemi, E. Rainero, Cross-Talk Between the Tumor Microenvironment, Extracellular Matrix, and Cell Metabolism in Cancer, Frontiers in oncology, 10 (2020) 239.
[66] H.J. Moon, J. Finney, T. Ronnebaum, M. Mure, Human lysyl oxidase-like 2, Bioorganic chemistry, 57 (2014) 231-241.
[67] M. Chen, J.L. Manley, Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches, Nature reviews. Molecular cell biology, 10 (2009) 741-754.
[68] B.L. Wu, G.Q. Lv, H.Y. Zou, Z.P. Du, J.Y. Wu, P.X. Zhang, L.Y. Xu, E.M. Li, Exploration of potential roles of a new LOXL2 splicing variant using network knowledge in esophageal squamous cell carcinoma, TheScientificWorldJournal, 2014 (2014) 431792.
[69] K. Mizuno, N. Seki, H. Mataki, R. Matsushita, K. Kamikawaji, T. Kumamoto, K. Takagi, Y. Goto, R. Nishikawa, M. Kato, H. Enokida, M. Nakagawa, H. Inoue, Tumor-suppressive microRNA-29 family inhibits cancer cell migration and invasion directly targeting LOXL2 in lung squamous cell carcinoma, International journal of oncology, 48 (2016) 450-460.
[70] K. Kamikawaji, N. Seki, M. Watanabe, H. Mataki, T. Kumamoto, K. Takagi, K. Mizuno, H. Inoue, Regulation of LOXL2 and SERPINH1 by antitumor microRNA-29a in lung cancer with idiopathic pulmonary fibrosis, Journal of human genetics, 61 (2016) 985-993.
[71] C. Reily, T.J. Stewart, M.B. Renfrow, J. Novak, Glycosylation in health and disease, Nature reviews. Nephrology, 15 (2019) 346-366.
[72] L. Oliveira-Ferrer, K. Legler, K. Milde-Langosch, Role of protein glycosylation in cancer metastasis, Seminars in cancer biology, 44 (2017) 141-152.
[73] E.P. Go, H.J. Moon, M. Mure, H. Desaire, Recombinant Human Lysyl Oxidase-like 2 Secreted from Human Embryonic Kidney Cells Displays Complex and Acidic Glycans at All Three N-Linked Glycosylation Sites, Journal of proteome research, 17 (2018) 1826-1832.
[74] Y. Wei, T.J. Kim, D.H. Peng, D. Duan, D.L. Gibbons, M. Yamauchi, J.R. Jackson, C.J. Le Saux, C. Calhoun, J. Peters, R. Derynck, B.J. Backes, H.A. Chapman, Fibroblast-specific inhibition of TGF-beta1 signaling attenuates lung and tumor fibrosis, The Journal of clinical investigation, 127 (2017) 3675-3688.
[75] P. Neumann, N. Jae, A. Knau, S.F. Glaser, Y. Fouani, O. Rossbach, M. Kruger, D. John, A. Bindereif, P. Grote, R.A. Boon, S. Dimmeler, The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2, Nature communications, 9 (2018) 237.
[76] H.E. Barker, J. Chang, T.R. Cox, G. Lang, D. Bird, M. Nicolau, H.R. Evans, A. Gartland, J.T. Erler, Correction: LOXL2-Mediated Matrix Remodeling in Metastasis and Mammary Gland Involution, Cancer research, 79 (2019) 5123.
[77] V. Brekhman, J. Lugassie, S. Zaffryar-Eilot, E. Sabo, O. Kessler, V. Smith, H. Golding, G. Neufeld, Receptor activity modifying protein-3 mediates the protumorigenic activity of lysyl oxidase-like protein-2, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 25 (2011) 55-65.
[78] J. Chang, M.M. Nicolau, T.R. Cox, D. Wetterskog, J.W. Martens, H.E. Barker, J.T. Erler, LOXL2 induces aberrant acinar morphogenesis via ErbB2 signaling, Breast cancer research : BCR, 15 (2013) R67.
[79] E.P. Cuevas, G. Moreno-Bueno, G. Canesin, V. Santos, F. Portillo, A. Cano, LOXL2 catalytically inactive mutants mediate epithelial-to-mesenchymal transition, Biology open, 3 (2014) 129-137.
[80] R. Schietke, C. Warnecke, I. Wacker, J. Schodel, D.R. Mole, V. Campean, K. Amann, M. Goppelt-Struebe, J. Behrens, K.U. Eckardt, M.S. Wiesener, The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia: insights into cellular transformation processes mediated by HIF-1, The Journal of biological chemistry, 285 (2010) 6658-6669.
[81] A. Millanes-Romero, N. Herranz, V. Perrera, A. Iturbide, J. Loubat-Casanovas, J. Gil, T. Jenuwein, A. Garcia de Herreros, S. Peiro, Regulation of heterochromatin transcription by Snail1/LOXL2 during epithelial-to-mesenchymal transition, Molecular cell, 52 (2013) 746-757.
[82] N. Herranz, N. Dave, A. Millanes-Romero, L. Pascual-Reguant, L. Morey, V.M. Diaz, V. Lorenz-Fonfria, R. Gutierrez-Gallego, C. Jeronimo, A. Iturbide, L. Di Croce, A. Garcia de Herreros, S. Peiro, Lysyl oxidase-like 2 (LOXL2) oxidizes trimethylated lysine 4 in histone H3, The FEBS journal, 283 (2016) 4263-4273.
[83] A. Martin, F. Salvador, G. Moreno-Bueno, A. Floristan, C. Ruiz-Herguido, E.P. Cuevas, S. Morales, V. Santos, K. Csiszar, P. Dubus, J.J. Haigh, A. Bigas, F. Portillo, A. Cano, Lysyl oxidase-like 2 represses Notch1 expression in the skin to promote squamous cell carcinoma progression, The EMBO journal, 34 (2015) 1090-1109.
[84] R.N. Kaplan, R.D. Riba, S. Zacharoulis, A.H. Bramley, L. Vincent, C. Costa, D.D. MacDonald, D.K. Jin, K. Shido, S.A. Kerns, Z. Zhu, D. Hicklin, Y. Wu, J.L. Port, N. Altorki, E.R. Port, D. Ruggero, S.V. Shmelkov, K.K. Jensen, S. Rafii, D. Lyden, VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche, Nature, 438 (2005) 820-827.
[85] H. Peinado, S. Lavotshkin, D. Lyden, The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts, Seminars in cancer biology, 21 (2011) 139-146.
[86] C.C. Wong, D.M. Gilkes, H. Zhang, J. Chen, H. Wei, P. Chaturvedi, S.I. Fraley, C.M. Wong, U.S. Khoo, I.O. Ng, D. Wirtz, G.L. Semenza, Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation, Proceedings of the National Academy of Sciences of the United States of America, 108 (2011) 16369-16374.
[87] L. Ma, C. Huang, X.J. Wang, D.E. Xin, L.S. Wang, Q.C. Zou, Y.S. Zhang, M.D. Tan, Y.M. Wang, T.C. Zhao, D. Chatterjee, R.A. Altura, C. Wang, Y.S. Xu, J.H. Yang, Y.S. Fan, B.H. Han, J. Si, X. Zhang, J. Cheng, Z. Chang, Y.E. Chin, Lysyl Oxidase 3 Is a Dual-Specificity Enzyme Involved in STAT3 Deacetylation and Deacetylimination Modulation, Molecular cell, 65 (2017) 296-309.
[88] X. Zhang, Q. Wang, J. Wu, J. Wang, Y. Shi, M. Liu, Crystal structure of human lysyl oxidase-like 2 (hLOXL2) in a precursor state, Proceedings of the National Academy of Sciences of the United States of America, 115 (2018) 3828-3833.
[89] X.H. Zhan, J.W. Jiao, H.F. Zhang, C.Q. Li, J.M. Zhao, L.D. Liao, J.Y. Wu, B.L. Wu, Z.Y. Wu, S.H. Wang, Z.P. Du, J.H. Shen, H.Y. Zou, G. Neufeld, L.Y. Xu, E.M. Li, A three-gene signature from protein-protein interaction network of LOXL2- and actin-related proteins for esophageal squamous cell carcinoma prognosis, Cancer medicine, 6 (2017) 1707-1719.
[90] D.A. Kirschmann, E.A. Seftor, S.F. Fong, D.R. Nieva, C.M. Sullivan, E.M. Edwards, P. Sommer, K. Csiszar, M.J. Hendrix, A molecular role for lysyl oxidase in breast cancer invasion, Cancer research, 62 (2002) 4478-4483.
[91] D.A. Abourbih, S. Di Cesare, M.E. Orellana, E. Antecka, C. Martins, L.A. Petruccelli, M.N. Burnier, Jr., Lysyl oxidase expression and inhibition in uveal melanoma, Melanoma research, 20 (2010) 97-106.
[92] H.M. Rodriguez, M. Vaysberg, A. Mikels, S. McCauley, A.C. Velayo, C. Garcia, V. Smith, Modulation of lysyl oxidase-like 2 enzymatic activity by an allosteric antibody inhibitor, The Journal of biological chemistry, 285 (2010) 20964-20974.
[93] V. Barry-Hamilton, R. Spangler, D. Marshall, S. McCauley, H.M. Rodriguez, M. Oyasu, A. Mikels, M. Vaysberg, H. Ghermazien, C. Wai, C.A. Garcia, A.C. Velayo, B. Jorgensen, D. Biermann, D. Tsai, J. Green, S. Zaffryar-Eilot, A. Holzer, S. Ogg, D. Thai, G. Neufeld, P. Van Vlasselaer, V. Smith, Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment, Nature medicine, 16 (2010) 1009-1017.
[94] S.A. Harrison, M.F. Abdelmalek, S. Caldwell, M.L. Shiffman, A.M. Diehl, R. Ghalib, E.J. Lawitz, D.C. Rockey, R.A. Schall, C. Jia, B.J. McColgan, J.G. McHutchison, G.M. Subramanian, R.P. Myers, Z. Younossi, V. Ratziu, A.J. Muir, N.H. Afdhal, Z. Goodman, J. Bosch, A.J. Sanyal, U.S. Gs, G.-U.-. Investigators, Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis, Gastroenterology, 155 (2018) 1140-1153.
[95] A.J. Muir, C. Levy, H.L.A. Janssen, A.J. Montano-Loza, M.L. Shiffman, S. Caldwell, V. Luketic, D. Ding, C. Jia, B.J. McColgan, J.G. McHutchison, G. Mani Subramanian, R.P. Myers, M. Manns, R. Chapman, N.H. Afdhal, Z. Goodman, B. Eksteen, C.L. Bowlus, G.-U.-. Investigators, Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results With Insights on the Natural History of the Disease, Hepatology, 69 (2019) 684-698.
[96] J.R. Hecht, A.B. Benson, 3rd, D. Vyushkov, Y. Yang, J. Bendell, U. Verma, A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma, The oncologist, 22 (2017) 243-e223.
[97] A.B. Benson, 3rd, Z.A. Wainberg, J.R. Hecht, D. Vyushkov, H. Dong, J. Bendell, F. Kudrik, A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma, The oncologist, 22 (2017) 241-e215.
[98] J.H. Hutchinson, M.W. Rowbottom, D. Lonergan, J. Darlington, P. Prodanovich, C.D. King, J.F. Evans, G. Bain, Small Molecule Lysyl Oxidase-like 2 (LOXL2) Inhibitors: The Identification of an Inhibitor Selective for LOXL2 over LOX, ACS medicinal chemistry letters, 8 (2017) 423-427.
[99] M.W. Rowbottom, G. Bain, I. Calderon, T. Lasof, D. Lonergan, A. Lai, F. Huang, J. Darlington, P. Prodanovich, A.M. Santini, C.D. King, L. Goulet, K.E. Shannon, G.L. Ma, K. Nguyen, D.A. MacKenna, J.F. Evans, J.H. Hutchinson, Identification of 4-(Aminomethyl)-6-(trifluoromethyl)-2-(phenoxy)pyridine Derivatives as Potent, Selective, and Orally Efficacious Inhibitors of the Copper-Dependent Amine Oxidase, Lysyl Oxidase-Like 2 (LOXL2), Journal of medicinal chemistry, 60 (2017) 4403-4423.
[100] H. Schilter, A.D. Findlay, L. Perryman, T.T. Yow, J. Moses, A. Zahoor, C.I. Turner, M. Deodhar, J.S. Foot, W. Zhou, A. Greco, A. Joshi, B. Rayner, S. Townsend, A. Buson, W. Jarolimek, The lysyl oxidase like 2/3 enzymatic inhibitor, PXS-5153A, reduces crosslinks and ameliorates fibrosis, Journal of cellular and molecular medicine, 23 (2019) 1759-1770.
[101] J. Chang, M.C. Lucas, L.E. Leonte, M. Garcia-Montolio, L.B. Singh, A.D. Findlay, M. Deodhar, J.S. Foot, W. Jarolimek, P. Timpson, J.T. Erler, T.R. Cox, Pre-clinical evaluation of small molecule LOXL2 inhibitors in breast cancer, Oncotarget, 8 (2017) 26066-26078.
