Jinni Wang1, 2, #, Ning Su3, #, Yu Fang1, 2, Shuyun Ma1, 2, Yuchen Zhang1, 2, Jun Cai1, 2, Qihua Zou1, 2, Xiaopeng Tian1, 2, Yi Xia1, 2, Panpan Liu1, 2, Zhiming Li1, 2, He Huang1, 2, Huiqiang Huang1, 2, Qingqing Cai1, 2, *
1. State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, P.R. China
2. Department of Medical Oncology, Sun Yat-sen University Cancer Center, Guangzhou, P.R. China
3. Guangzhou Chest Hospital, Department of Oncology
# These authors contributed equally.
*Corresponding Author: Dr Qingqing Cai, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, 651 Dong Feng RD East, Guangzhou 510060, P.R. China.
Email: caiqq@sysucc.org.cn; Telephone: 86-20-87342823; Fax: 86-20-87342823.
Abstract
Background: Peripheral T-cell lymphoma (PTCL) is characterized by a poor clinical outcome. Chidamide, an oral novel histone deacetylase inhibitor, has been approved for the treatment of relapsed or refractory PTCL in China. Aim: In this study, we set out to evaluate whether the survival outcome of chemotherapy combined with chidamide were superior to that of chemotherapy, and investigated clinical outcome and prognostic factors influencing progression and survival in patients with newly diagnosed PTCL.
Methods: 104 of patients diagnosed with newly diagnosed PTCL and managed at Sun Yat-sen University Cancer Center were enrolled in our studies. Based on the frontline treatment regimens, patients were divided into two groups, including chemotherapy (ChT) and chemotherapy combined with chidamide (ChT+C). Survival curves were plotted using the Kaplan-Meier method. A log-rank test was used for a univariate analysis of survival. Multivariate analyses of Cox’s proportional hazard regression were used to calculate the hazard ratio (HR) in 95% confidence intervals (95% CI) and determine independent prognostic factors. Subgroup analysis was performed to assess survival outcome of treatment groups. Interaction tests were performed on the patients enrolled in our study.
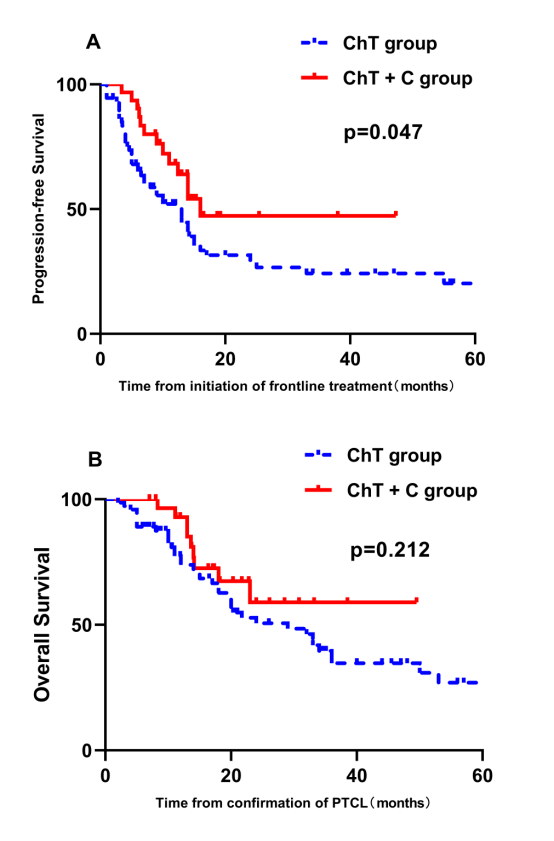
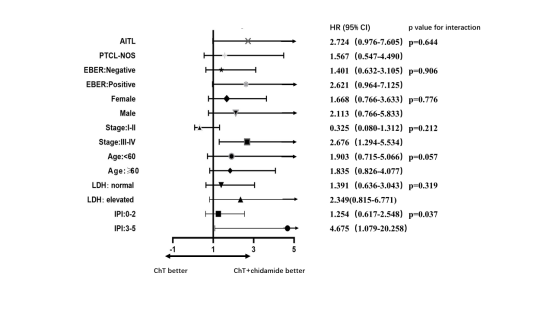
Results: ChT+C group had a significantly better progression-free survival (PFS) compared with ChT group (p=0.047, Fig 1A). However, there was no significant difference in overall survival (OS) between these two groups (p=0.212, Fig 1B). Consistent with previous data, our prognostic factor analysis demonstrated the patients with high IPI scores had progressively inferior PFS and OS (p=0.028 and 0.003, respectively) (Fig 2A and Fig 2B). With further investigation of the effects of the components of the IPI and frontline treatments on PFS by univariate and multivariate analysis, frontline treatment regimen was identified as an independent prognostic factor for PFS in PTCL patients (95%CI 1.015 to 3.487, p=0.045). The forest plot indicated that the HR value for patients with high IPI scores (3-5) was 4.675 (95%CI 1.079 to 20.258, Fig 3). A test of interaction between IPI and treatment was statistically significant (p = 0.037, Fig 3), indicating longer PFS for patients treated with ChT+C in the subgroup of high IPI scores.
Conclusion: Overall, the combination of ChT and chidamide may be an effective treatment strategy for newly diagnosed PTCL patients. Treatment-related toxicities characterized by hematological events were manageable and reversible.